Two Kinds Of Particles Mac OS
Two Kinds Of Particles Mac OS
Mac OS X & macOS names. As you can see from the list above, with the exception of the first OS X beta, all versions of the Mac operating system from 2001 to 2012 were all named after big cats.
- Version for Mac OS Description The citizens of Houndspoint have always loved and trusted their canine companions, but a recent wave of coordinated attacks by a pack of strange dogs has thrown the snowy mountain town into a state of fear.
- Maya was developed by Alias and is released for the Microsoft Windows, Linux, IRIX, and Mac OS X operating systems. The latest version of Maya, version 8.0, was released in August 2006. 6.5 was the last version that supported IRIX, due to the platform's declining popularity in recent years.
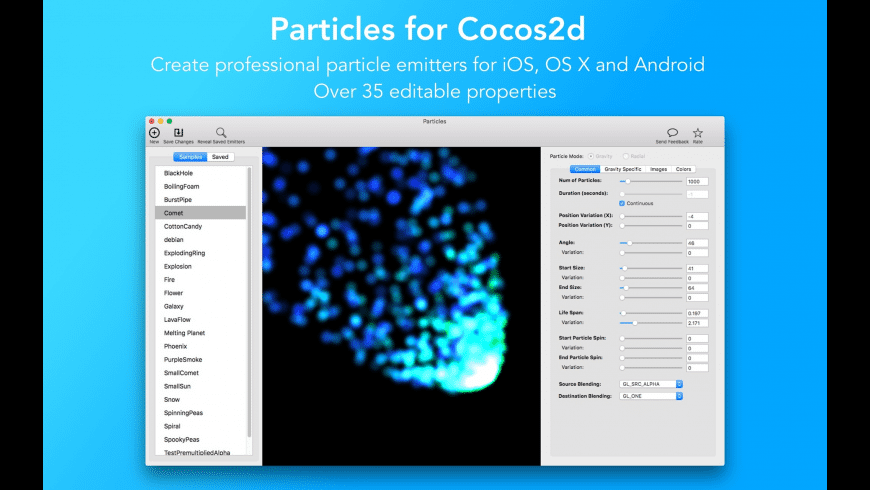
- ParticleShop-a powerful new plug-in for Adobe Photoshop, powered by Corel Painter-creates one-of-a-kind effects.Experience living grab-and-go particle brushes that are easy to use with a pressure sensitive tablet, touchscreen, or mouse, and which allow you to artistically enhance photos, designs, and illustrations with strokes of genius.
- The Kindle app gives users the ability to read eBooks on a beautiful, easy-to-use interface. You may also read your book on your phone, tablet and Kindle e-Reader, and Amazon Whispersync will automatically sync your most recent page read, bookmarks, notes, and highlights, so you can pick up right where you left off.
ParticleShop--a powerful new plug-in for Adobe Photoshop, powered by Corel Painter--creates one-of-a-kind effects. Experience living grab-and-go particle brushes that are easy to use with a pressure sensitive tablet, touchscreen, or mouse, and which allow you to artistically enhance photos, designs, and illustrations with strokes of genius.
Features
- Explore an array of 11 imaginative brushes--including Debris, Fabric, Fine Art, Fur, Hair, Light, Space, Smoke, and Storm--that will produce awe-inspiring results in no time. No experience, customization, or time-consuming learning is required. Plus, the plugin is only the beginning! Enjoy infinite inspiration with our extra brush packs available for purchase.
- Evolutionary Brush Technology Powered by Painter - Leave the physics to us; no static stamps here! Experience the fluid strokes of living grab-and-go brushes that spring, flow, gravitate, and glow.
- Intuitive User Experience - Craft your visual masterpiece with the Adobe Photoshop brush plugin, built to perform with a pressure sensitive tablet, touchscreen, or mouse.
- No Upgrade Required - Create with the software and hardware you currently own; nothing more to buy or add.
- Infinite Expression - Achieve mind-blowing effects with physics-based brushes that will follow your every move. Design like a pro with chaotic storms, hairy creatures, flowing gowns, or sci-fi superheroes.
The key difference between atoms and particles is that atoms are small units made of several particles, whereas particles are minute portions of matter.
An atom is the smallest unit of all matter. In the past, people thought that the atom was the smallest thing that exists and we cannot break it down further. But according to recent research studies, the atom is made of several small particles called subatomic particles. However, the term particle in chemistry refers to any small localized object which has physical properties such as volume, density and mass.
CONTENTS
1. Overview and Key Difference
2. What are Atoms
3. What are Particles
4. Side by Side Comparison – Atoms vs Particles in Tabular Form
5. Summary
What are Atoms?
Atoms are the smallest particles of a chemical element that can exist. Therefore, it is the smallest unit of matter, and a certain atom represents the properties of the chemical element to which it belongs. All gases, solid matter, liquids and plasma contain atoms. These are very minute units; typically the size is around 100 picometers.
When considering the structure of an atom, it contains a nucleus and electrons moving around the nucleus. The atomic nucleus is made of protons and neutrons (and there are some other subatomic particles as well). Typically, the number of neutrons, protons and electrons are equal to each other, but in the case of isotopes, the number of neutrons is different from that of protons. Around 99% of the atom’s mass is centred in the nucleus because the mass of an electron is almost negligible. Among these subatomic particles, a proton has +1 charge, and an electron has -1 charge while neutron has no charge. If the atom has equal numbers of protons and electrons, then the overall charge of the atom is zero; lack of one electron results in a +1 charge and gain of one electron gives -1 charge to the atom.
The number of protons in the atom decides the chemical element to which the atom belongs. That means; a certain chemical element has a certain number of protons in their atoms.
Furthermore, atoms take part in chemical bonding via gaining, removing or sharing their electrons in the outermost orbitals. The formation of chemical bonds results in the formation of chemical compounds or molecules. Most of the physical changes in nature occur due to the ability of these atoms to associate and dissociate.
What are Particles?
A particle is a minute portion of matter. It is a small localized object which has properties such as mass, volume and density. The size of particles may vary from subatomic particles such as electrons to microscopic particles such as molecules and even to macroscopic particles, i.e. granular material.
Figure 02: Powder Contains Macroscopic Particles
Generally, we use the term particle for three major sizes; macroscopic, microscopic and subatomic particles. Macroscopic particles are larger than atoms and molecules and are visible to the naked eye. Examples include powder and dust particles. Microscopic particles are invisible to the naked eye but visible through microscopes. It mainly includes particles with sizes ranging from atoms to molecules. Examples include nanoparticles and colloidal particles. Subatomic particles are the components in atoms: protons, neutrons, electrons, etc.
What is the Difference Between Atoms and Particles?
The key difference between atoms and particles is that atoms are small units containing several particles, whereas particles are minute portions of matter. There are three different types of particles as macroscopic, microscopic and subatomic particles. When considering different types of atoms, they belong to different chemical elements depending on the atomic numbers. The size of an atom is around 100 picometers while the size of a particle varies from subatomic particle to macroscopic particles.
The below infographic summarizes the difference between atoms and particles in tabular form.
Summary – Atoms vs Particles
Atoms are small units of matter which contain several particles; we call them subatomic particles. However, the term particle refers to any small object. Therefore, the key difference between atoms and particles is that atoms are small units made of several particles, whereas particles are minute portions of matter.
Reference:
1. “Atom.” Wikipedia, Wikimedia Foundation, 25 May 2019, Available here.
2. Nordquist, Richard. “Particle Definition and Examples in English Grammar.” ThoughtCo, Dec. 6, 2018, Available here.
Image Courtesy:
1. “Atom-struc” By AhmadSherif – Own work (Public Domain) via Commons Wikimedia
2. “2998946” (CC0) via Max Pixel
Two Kinds Of Particles Mac Os Sierra
Related posts:
Two Kinds Of Particles Mac OS
